Optimize Global Medical Communications
SCIMAX MI is an intelligent global medical information management system that is leveraged by pharma, biotech, medical device, and contact center companies to collect and respond to medical inquiries and to report potential product complaints and adverse events for all types of therapeutic areas including specialty and generic products.

Ensure global compliance to GxP standards, HIPAA, EU-GDPR, and other regulations
SCIMAX MI increases your company’s global compliance with country-specific privacy and security rules including data protection policies, data encryption, and Right to Forget.
The electronic records and electronic signatures defined and maintained within SCIMAX MI meet GxP regulatory compliance including FDA 21 CFR Part 11 requirements.
SCIMAX MI has an audit trail at case level, response level, report level, and administration level.
Reduce timelines and redundancy in responding to requests.
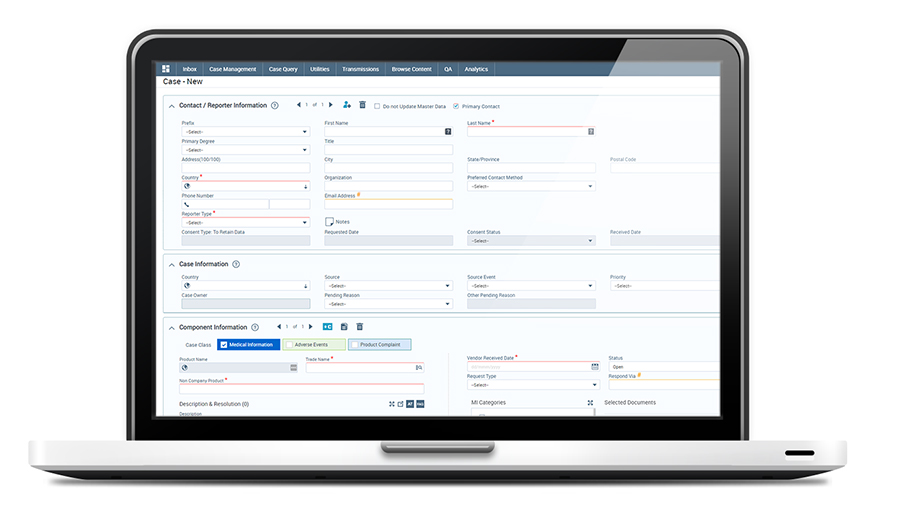
SCIMAX MI offers multi-channel case intake with intuitive workflows and rapid case creation features including duplicate search CRM contacts lookup, zip code lookup, and nonlinear data entry.
Multiple requests can be captured within a single case and responded to within minutes using the pre-configured product and reporter specific FAQs, auto-selected standard response documents, and templatized response packages.
The built-in medical communications inbox with preconfigured email templates saves time on manual drafting of emails and eliminates the cumbersome process of including source attachments and reports while processing adverse events and product complaints.

See Scimax MI in Action -
Request a demo today

Highly configurable, SCIMAX MI can easily be tailored to facilitate your medical communications business processes from local to regional to global levels.
The powerful Admin Console allows you to customize workflows, easily add/edit users, create custom forms, and set different permissions for your internal groups i.e., content creators, content approvers, or executive management.
Create site- or region-specific workflows for processing medical information requests, escalating different types of inquiries, and transmitting adverse events and product complaints to other business groups.
Leverage robust case search options to filter, preview, and route cases to different user groups and users.
SCIMAX MI allows fields to be renamed and flex-fields added to tailor the system to meet your business requirements.
Automate your business processes with SCIMAX MI integration.
With internal correspondence management, case level communications can be sent to cross-team members and customers directly within SCIMAX MI.
Our Self-Service and Collaboration Portals work with the application to improve efficiency and potentially decrease costs by allowing healthcare providers to independently search and retrieve approved content.
An intuitive web form can be setup within the portals for intake of medical information requests along with potential adverse events and product complaints. The data collected on the web forms can be automatically imported to SCIMAX MI to create a new case.
SCIMAX MI offers powerful APIs to integrate with your CRMs, content repositories, data analytics tools, and safety applications.


The SCIMAX MI platform includes a robust suite of quality assurance, content management (CM), and analytics features.
SCIMAX MI QA is a powerful tool for ensuring your team is providing the highest quality and most accurate responses to inquiries. The built-in data validation and business rules can be configured to ensure that all case processing activities are performed with the highest quality as per your organization's process requirements.
With MS Word look and feel, SCIMAX MI CM can easily manage all product specific or site-specific FAQs, standard response letters, cover letters, enclosures, package inserts, and posters.
Prepare, share, and export case data listings, interactive visual dashboards, and summary metric reports using the SCIMAX MI built-in reporting and analytics engine.


